New nanogenerator might set energy-generating car wheels in motion
Xudong Wang’s team has developed a new way to harvest energy from rolling tires. The researchers used toy cars during the initial trials
Cars are one of mankind’s most revolutionary creations. But just like with the iPhone, space travel or Wi-Fi, there’s always room for improvement. In the eyes of a team of University of Wisconsin-Madison engineers, one of the more promising ways automotive technology might be improved upon lies in the energy wastage caused by friction as tires roll across the road. Armed with special nanogenerator and a toy Jeep, the researchers have demonstrated that this power can be captured and turned into electricity, a development that could bring about better fuel efficiency in the full-sized cars of the future.
According to Xudong Wang, associate professor of materials science and engineering at the University of Wisconsin, the friction created as a car’s tires run over the ground accounts for approximately 10 percent of the vehicle’s fuel usage. For him and PhD student Yanchao Mao, this presents a big opportunity to improve efficiency, so for the last year or so they have been building a device to tackle the problem.
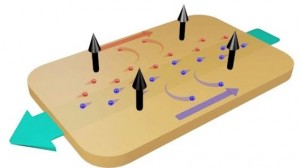
Their work looks to harness the electrical charge that is created when certain materials come into contact with one another, much like what happens when you run a comb through your hair. This is known as the triboelectric effect and has been used in the early-stage development of promising technologies like electricity-generating touchscreens and clothing.
Not to be confused with the approach taken by Goodyear, which in March unveiled a concept tire that turns heat and motion into electricity using a fishnet pattern of thermo/piezoelectric material, Wang’s solution sees an electrode built into a section of the tire. As the wheel spins and this part of the tire comes into contact with the ground, the charge created by the friction causes electrons to move, in turn generating electricity.
To bring this new source of electricity to life, the team equipped the toy Jeep with LED lights. As the car moved forward, enough power was created to cause the lights to flash on and off, suggesting that this hitherto wasted energy could actually be captured and put to use.
Interestingly, the researchers also found that the amount of energy the system was able to produce was proportionate to both the weight of the vehicle and the speed at which it was traveling. Wang estimates that the solution could offer approximately a 10 percent increase in the average vehicle’s gas mileage.
References:http://www.gizmag.com/